about us
AHRP
Our Mission
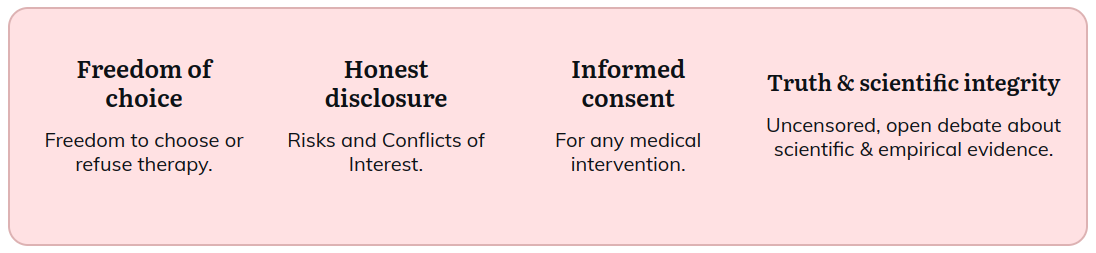
The Alliance for Human Research Protection (AHRP) is a national network of lay people and professionals who are committed to upholding the humanitarian values and ethical standards of medicine enshrined in the Hippocratic Oath: “First, do no harm”; the Nuremberg Code (1947): “The Voluntary informed consent of the human subject is absolutely essential”; and the UNESCO Universal Declaration on Bioethics and Human Rights (2005): “Any preventive, diagnostic and therapeutic medical intervention is only to be carried out with the prior, free and informed consent of the person concerned, based on adequate information.”
Newsletter
On Substack
Receive our monthly Newsletter with the latest News highlighting Geopolitics, Individual and Public Health, Propaganda, Global Agendas, Human Rights, Solutions and Building Resilience
Subscribe Today
Dignified reporting, profound research for you to come to your own conclusions
2’600+
Posts
100%
Proven Quality
15’000
Subscribers
AHRP’s mission is to ensure that the moral right of voluntary medical decision-making is upheld. To accomplish that mission, we engage in an educational campaign, providing relevant factual information including ethical, legal, technical reports, and media reports. We endeavor to counter widely disseminated false claims that exaggerate the benefits of medical interventions, while minimizing risks.
Our educational efforts are directed at both professionals and the lay public, who may be unaware of a body of suppressed scientific evidence that refutes false promotional claims for drugs; including antidepressants, antipsychotics, statins; medical devices; and vaccinations, including those for Hepatitis B, HPV, and the flu.
Our goal is to empower citizens with accurate information so that they can exercise their right to informed consent to medical research and medical procedures within clinical care. As a citizens’ watchdog group, AHRP brings to public attention specific unethical violations of informed consent wherever they occur.
subscribe to newsletter
Position Statement Regarding Essential Research Safeguards for Human Subjects:
Medical research is not standard medical care: research involves a high level of uncertainty and, therefore, risk. In standard care the selection of a treatment and the dose/or level of an intervention is based on the physician’s clinical judgment of what the best therapeutic choice is to meet an individual patient’s clinical condition and need.
Within the research arena, treatment is determined by a study protocol that seeks to resolve uncertainty and contribute to generalizable knowledge. The treatment provided within the research context is not necessarily determined by a medical doctor to be in the best interest of the individual patient/subject. Human subjects enrolled in research will always be exposed to risk. At a minimum, they may receive a placebo or a less effective treatment than they would in standard care. In the worst case, human subjects may be irrevocably harmed and may even die due to the treatment being tested, the experimental procedures used, or a needed treatment withheld.
Human subjects of research have no guarantee that if they incur an injury during an experiment, they will receive medical treatment without cost, nor that their family will be compensated for a permanent disability or death. Many clinical trials that are conducted by or on behalf of pharmaceutical companies are performed off-shore, mostly in very poor, illiterate populations.
The Alliance for Human Research Protection recommends 5 Essential Safeguards for ALL human subjects in clinical trials.
- Informed consent should never be abrogated for any research involving human subjects, under any circumstances, in any country, regardless of local standards and regardless of the type of research. For this safeguard to be meaningful, researchers must fully disclose the known and foreseeable risks, and the person who is being asked to be a subject, must be capable of understanding potential risks and benefits, before being asked to consent.
- Patients who volunteer to be human subjects must be fully informed about all the foreseeable risks—including the risks associated with randomization, which requires patients to be on a fixed dose, level or combination of a tested treatment compared to usual treatment in current care.
- Strict treatment protocols may result in suboptimal patient outcomes, whether the research is classified as “comparative effectiveness research” or any other classification, in which a new treatment or a fixed dose of a treatment must be tested against another fixed dose. There must always be an option to diverge from the protocol when a patient’s life or health is endangered by strict adherence to it.
- All clinical trials must include a true standard of care comparator arm, to protect human beings from exploitation in poorly designed clinical trials; and to ensure that treatment practices that fail to improve on current standard of care are not adopted as practice guidelines based on flawed comparative trial designs.
- There are currently no mandatory licensure requirements for researchers conducting research on human subjects. AHRP recommends that those who conduct medical research involving human beings should be required to undergo rigorous training on how to minimize risks and demonstrate proficiency in ethical and clinical standards, BEFORE they can be licensed to conduct medical experiments in human beings.
- The Alliance for Human Research Protection is a nonprofit, tax-exempt educational organization under Section 501 C-3 of the Internal Revenue Code.
- Our board of directors are all unpaid volunteers who donate both time and other resources to the organization.
- Aid Our Efforts: Make a tax-deductible contribution.
