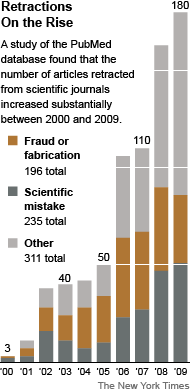
Fraudulent Science: What’s Retracted, What’s Not
Even if retracted, published clinical trial reports that misrepresent findings, withhold negative data, or make false, or unsubstantiated claims have done irreparable damage. AHRP calls upon all medical journals to adopt a publication policy requiring submission of the sponsor’s formal Clinical Study Report to accompany articles about clinical trial findings.