Bearing Witness to Genocide Yet Again
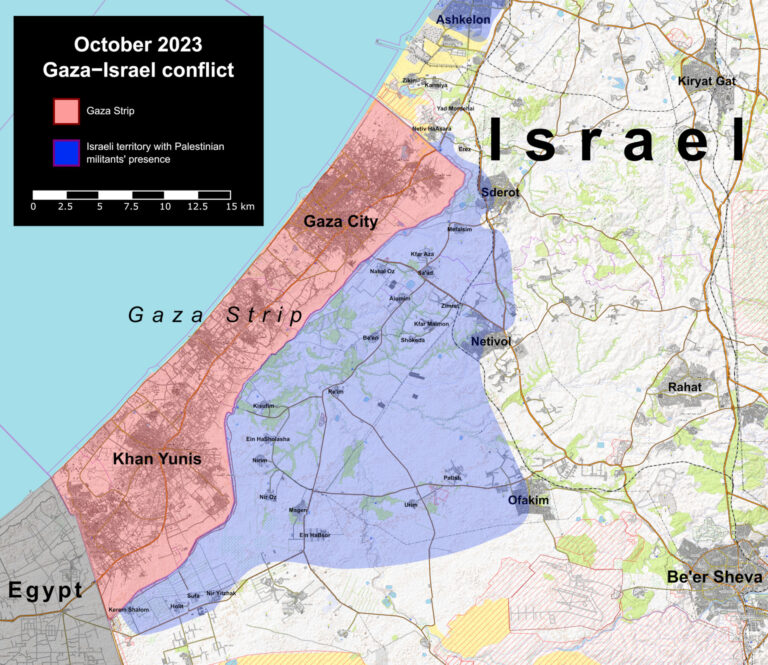
When will they ever learn? Oh, when will they ever learn? Push Back Against Palestinian Ethnic Cleansing Hundreds of Rabbis, Jewish American scholars, celebrities, and artists took out a full-page ad in The New York Times denouncing ethnic cleansing in Gaza. The ad appeared on February 13th on page…