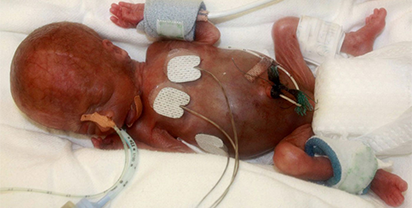
Doctors Deceived, Premature Infants’ Lives Sacrificed
Extremely low weight premature infants have an approximately 20% mortality rate —if treated with the best current practice. These babies’ lives are at serious risk.
A legitimate research question: what is the appropriate amount of oxygen an essential therapy for fragile premature infants? Too much oxygen therapy could cause blindness and to little causes death.
 An illegitimate research protocol: the infants in the government-sponsored SUPPORT research protocol were extremely low weight premature infants who were randomized to be maintained at either high oxygen saturation SOP2 (91-95%) or low (85-89%) oxygen saturation. In current practice, the full range of oxygen saturations has been used; the level usually selected to be maintained is based on trying to give the minimal oxygen necessary to prevent blindness while maintaining adequate oxygen delivery so the neonate will survive.
An illegitimate research protocol: the infants in the government-sponsored SUPPORT research protocol were extremely low weight premature infants who were randomized to be maintained at either high oxygen saturation SOP2 (91-95%) or low (85-89%) oxygen saturation. In current practice, the full range of oxygen saturations has been used; the level usually selected to be maintained is based on trying to give the minimal oxygen necessary to prevent blindness while maintaining adequate oxygen delivery so the neonate will survive.
The most disturbing fact that has emerged about the neonatal SUPPORT trial design is that the pulse oximeter readings relied upon by treating neonatologists to determine the infants’ need for supplementary oxygen, was intentionally altered to give either false high or false low SOP2 values. See the SUPPORT protocol : 
Whatever the motive (supposedly to maintain the blind) this is unprecedented in the annals of academic research. Consider the risks for these premature infants—if given too much oxygen, they will likely become blind, if given too little oxygen, they are likely to die. The neonatologists treating the infants during the experiment were given wrong information upon which they relied to make life and death decisions about what level of oxygen to deliver to these critically ill infants.
None of the infants in the SUPPORT experiment received standard care by a fully informed neonatologist who would be provided accurate information from the pulse oximeter which allows the neonatologist to then select from a full range of oxygen saturations the best level to maintain the neonate—depending whether the treating physician thought blindness or death was a greater risk for the particular infant.
In the published article , the SUPPORT researchers acknowledge “the increase in mortality when restrictive oxygen supplementation was used in the 1950s and 1960s…”
They failed to disclose this vital information in the protocol submitted to the reviewing IRB committees, and in the consent forms given to parents. In the informed consent document they state that lowering oxygen level may prevent blindness but fail to discuss the corollary that if you increase oxygen level in the other arm of the study blindness may increase. Moreover there is no information given to parents that the neonatologists would be using modified pulse oximeters that provide false readings either too high or too low.
Evidently, the investigators thought it was important to discuss the risk of having a pulse oximeter pad on the infants’ body which they would have had anyway (even if not in the trial) but was not important to inform the parents the pulse oximeter used in in the study would be jerry-rigged to give false readings. I can understand why—I might agree to the research if told I would have a harmless pad on my infants’ body but not if the neonatologist was going to treat my critically ill infant based on false oxygen readings. The experiment confirmed the long known increased risk of death in infants consigned to the restricted low oxygen group but its unknown if the false information given the neonatologist further increased their risk of death even more than just getting to low an oxygen level.
We identify some of the egregious ethical violations that render this experiment unethical: for starters, bedrock medical ethics principles—“First, do no harm”—and core Federal statutory protections—“risks to subjects are minimized” and full disclosure of known and foreseeable risks were violated.
- According to the findings by the Office of Human Research Protection (OHRP), for many of those infants, the level of oxygen they had received in the experiment was different from what they would have received had they not participated in the study:
“According to the study design, on average, infants assigned to the upper range received more oxygen than average infants receiving standard care, and infants assigned to the lower range received less. Thus the anticipated risks and potential benefits of being in the study were not the same as the risks and potential benefits of receiving standard of care. For the infants assigned to the upper range, based upon the premises of the researchers, the risk of ROP [retinopathy of prematurity] was greater, while for the infants assigned t
o the lower range the risk of ROP was lower. And, as described above, there were also risks relating to neurological development and possibly death.”
OHRP notes that at the time of the experiment, “it was well recognized that changing a premature infant’s amount of exposure to oxygen could have an impact on a number of important health outcomes, including the development of severe eye disease (and possibly blindness); reduced neurologic development, including brain damage, chronic lung disease; and death.”
2. The experiment was conducted without a standard care control group whose oxygen is titrated according to the individual infant’s need. A control group is essential for monitoring the safety of fixed oxygen regimens under investigation—and is necessary for assessing whether the tested treatment is better than current practice. Without a current practice control, the trial provides no useful information for future care.
3. An unprecedented ethical and scientific deviation: “Pulse oximeter readings were altered between values of 85% to 95%…The entire range of actual SPO2 is altered either higher or lower value.”
Thus, treating neonatologists who cared for the infants during the experiment were deliberately misinformed, given inaccurate information about the infants’ oxygen saturated blood levels. See, p. 17 and 18 of the PROTOCOL .
- Informed Consent requirements were largely violated:
- Well documented risks were not disclosed in the protocol nor in consent documents; Prospective parents were misled to believe that their infant would receive standard treatment and therefore would not incur risks;
- Parents were led to believe that “there may be benefits to your child including…a decrease in the need for eye surgeryas a result of exposure to oxygen.”
- Parents were not informed about risks associated with randomization to either high or low oxygen levels;
- The consent form did not identify a single specific risk relating to the randomizing infants to high or low oxygen such as:by restricting oxygen—as was the case in one randomized arm of the experiment—the risk of death is increased;
- Consent was sought from women in labor who most likely had been given opiods or sedatives—they were hardly in a stable emotional or mental state to make competent, sound judgments about research.
The outcome of the SUPPORT trial conducted at 23 medical centers from 2005 to 2009, as reported in the NEJM, 2010:
“an increase in mortality and a substantial decrease in severe retinopathy among survivors….like most non-randomized studies, our trial confirmed that lower target rates of oxygenation result in a large reduction in the incidence of severe retinopathy among survivors. However, our data suggest that there is one additional death for approximately every two cases of severe retinopathy that are prevented.”
The medical establishment has circled the wagons to defend this illegitimate research paradigm whose subjects are not freely consenting volunteers; a paradigm that results in lives of critically ill patients in hospital intensive care units to be sacrificed in the interest of research expediency. The leading defender of the experiment is Dr. Jeffrey Drazen, editor in chief of the New England Journal of Medicine, publisher of numerous experiments whose ethics and scientific integrity are hotly disputed.
For example, Johnson & Johnson’s Vioxx trial, VIGOR, whose deaths were concealed in NEJM report;
and the ARDS-ALI lung ventilation experiment, which Dr. Drazen defended, arguing in an editorial in the NEJM that no one—not even OHRP, the Federal oversight agency whose responsibility is to ensure research involving human subjects complies with Federal ethics requirements, has standing to question the scientific or ethical merit of trials approved by NIH:
“The NIH has an established record of designing, reviewing, and monitoring clinical trials; it has the scientific expertise and the staffing to assume responsibility for the appropriateness of trial design. Concern about the safety of human subjects that derives from the design of the trial is an issue for the NIH.” Dr. Drazen was a member of the NIH advisory committee that approved the ARDS-ALI experiment.
A lawsuit has been filed against the University of Alabama on behalf of 5 premature infants who were enrolled at that University in the SUPPORT experiment; the parents claim negligence and failure to inform them about the risks. The suit seeks class-action status.
Experiments such as SUPPORT—and similarly designed experiments conducted on critically ill patients in intensive care units—represent an extreme breakdown in medical research ethics. Incapacitated patients in these experiments were unable to exercise the right to informed consent; they were knowingly put at increased risk of serious harm.
See: https://ahrp.org/cms/content/view/25/81/
See also: critiques by Robert Kacmarek, MD, Professor, Harvard University and Head of Respiratory Care Services, Mass. General Hospital;
Drs. Eichacker, Banks and Natanson , Senior scientists, Critical Care Medicine, National Institutes of Health.
This case sheds light on the profound chasm between the patient-centered objectives of practicing critical care specialists, and the objective of research whose protocols are not guided by ethical principles and patient / subject’s best interest.
In routine critical care practice, treatment decisions about the level (or dose) of therapy administered to critically ill patients— such as premature infants and lung-injured patients who are struggling to live—are based on several factors requiring a physician’s expertise and judgment. Critically ill patients often require titrated treatment, and continued monitoring and treatment adjustments which are determined by the individual patient’s need. Great care must be taken because any error in their treatment can be lethal; the objective is to reduce the number of deaths and severe eye disease in premature infants. Critical care medicine cannot be pigeon holed by one-size-fits all therapy favored by those who formulate practice guidelines that supplant physician’s professional judgment.
Vera Sharav