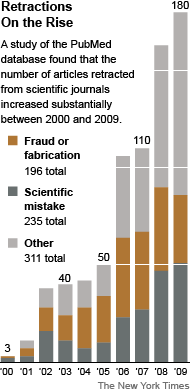
Anthrax Vaccine Trial Ethics? Science?
A strong case for the anthrax vaccine trial would be made “if the community that’s most supportive of moving this forward would volunteer their own children for the study.”Amy Gutmann,Chair, Presidential Commission for the Study of Bioethical Issues.