Avandia, a Case of Deja Vu all over again
Prozac Suicide Risk – CNN Posts long concealed Eli Lilly Internal Documents Tue, 4 Jan 2005 An internal company document titled Activation and Sedation in Fluoxetine Clinical Trials dated 11/8/1988, provides insight into how marketing divisions of pharmaceutical companies rationalize lethal adverse drug effects. Of note: the terminology in 1988…
Sen Schumer asks FDA to place hold on Paxil for kids Fri, 13 Jun 2003 Senator Charles Schumer held a press conference in New York to announce that he had written to Mark McClellan, FDA Commissioner to investigate the adverse effects of Paxil, the SSRI antidepressant on children and teens,…
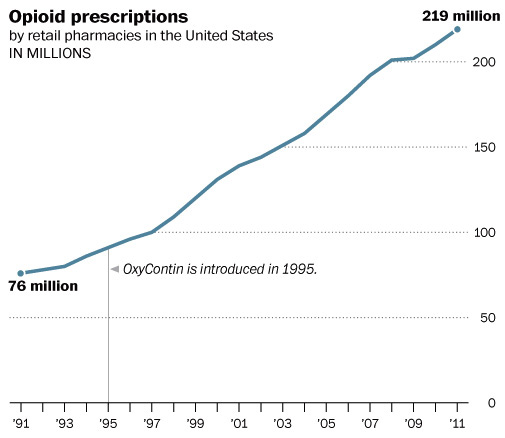
“You could say these marketing tactics are merely concerning. But I think of them as satanic. What the data are telling us is that these drugs are ruining people’s lives,” said Phillip Prior, MD
GlaxoSmithKline Reverses Decade of Denial: in Clinical Trials Paxil Triggered Significantly More Suicide Attempts in Adults than Placebo
TONIGHT ABC “PRIMETIME LIVE” INVESTIGATION reveals evidence that drug makers suppressed antidepressant info Thu, 9 Dec 2004 At least 100 children have committed suicide while on an SSRI antidepressant, hundreds more have attempted suicide. Tonight at 10:00 PM, ABC Prime Time Live will reveal internal company documents that show how…
Psychiatry’s leadership is scrambling and fumbling in its effort to explain why it’s collusion with industry for pay is okay.