FDA Scientists Collide with FDA Managers Re: Safety Issues
Since 1995 FDA scientists have been at odds against FDA managers who routinely override sientists’ safety concerns to approve dubious prescription drugs.
Since 1995 FDA scientists have been at odds against FDA managers who routinely override sientists’ safety concerns to approve dubious prescription drugs.
Autism: Vaccine Injury Compensation Program (VICP) data.
List of FDA-licensed prescription drugs withdrawn from US market for safety issues:
Between 1973 and 1991 (18 years) —16 drugs were withdrawan; Between 1992 and 2011 (19 years)—28 drugs were withdrawn.
Dr. J. Paul Muizelaar, Chairman of the department of neurological surgery, and Dr. Rudolph J. Schrot, injected bacteria into the head wounds of terminally ill patients, calling it "innovative treatment."

“Prescription drug therapy stands as one of the most significant perils to health resulting from human activity.” What’s the FDA doing to stem the tide of this preventable epidemic?

America’s healthcare system is riddled with financial conflicts of interest. Business interests and commercial priorities undermine the precautionary principle of Medicine, the therapeutic mission of Medicine, the scientific integrity of Medicine. Government oversight agencies and Congress have subordinated the public interest for $$$. As a result, America’s healthcare delivery system is the third leading cause of death.
A strong case for the anthrax vaccine trial would be made “if the community that’s most supportive of moving this forward would volunteer their own children for the study.”Amy Gutmann,Chair, Presidential Commission for the Study of Bioethical Issues.
At a minimum, the practice of responsible medicine requires that physicians who prescribe drugs whose known severe adverse side effects are likely to cause their patients irreversible harm, requires that those physicians follow monitoring guidelines to ensure their patients’ safety.
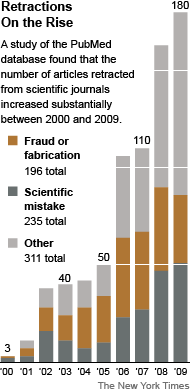
Even if retracted, published clinical trial reports that misrepresent findings, withhold negative data, or make false, or unsubstantiated claims have done irreparable damage. AHRP calls upon all medical journals to adopt a publication policy requiring submission of the sponsor’s formal Clinical Study Report to accompany articles about clinical trial findings.
Even as the corruption of science is no longer a dirty little secret, but is in plain sight, no one is doing anything tostop it. April 16, 2012 A Sharp Rise in Retractions Prompts Calls for Reform By CARL ZIMMER In the fall of 2010, Dr. Ferric C. Fang…
An urgent call for a debate about the ethics of data secrecy. Absent the humanitarian raison d’etre for enrolling in a clinical trial, no human being should be put at any–even minimal risk–without adequate compensation as a laborer and the protection of Workmen’s Compensation insurance.
On Friday, April 6, the FDA approved another of Eli Lilly’s "breakthroughs" whose clinical value is questionable (at best).