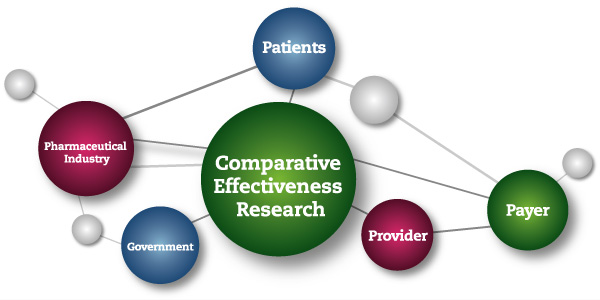
Who decides if it’s treatment or a medical experiment?
Current worrisome trends U.S. government and academic bioethicists and institutional ethics review boards (IRB) have expunged the explicit honest terminology of the Nuremberg Code: “experiment” has been replaced by the innocuous term “study”; “human subject” is being replaced by “participant”; and the foremost ethical mandate — “the voluntary, informed consent…