
Similar Posts
OHRP Found NHLBI Lung Experiments Violated Informed Consent
OHRP Found NHLBI Lung Experiments Violated Informed Consent Mon, 7 Jul 2003 On July 3 the federal Office of Human Research Protections (OHRP) issued a stinging indictment of the research review and approval process at some of the nation’s most prestigious research institutions–including the National Heart Lung and Blood Institute…
 AHRP Speaks Out | Board of Directors | Honest Science | Miscellaneous | Newsflash | Presentations by AHRP/Board Members | Presentations of Interest | Publication Integrity | Research Integrity
AHRP Speaks Out | Board of Directors | Honest Science | Miscellaneous | Newsflash | Presentations by AHRP/Board Members | Presentations of Interest | Publication Integrity | Research IntegrityDr. Meryl Nass will Confront the Maine Board of Licensure in Court on Oct. 11, 2022 at 1:00 PM
The entire hearing can be watched on video. In November 2021, The Maine Board of Licensure in Medicine suspended Dr. Nass’ license to practice medicine without a hearing. What’s more, the board added insult to injury by demanding that she undergo a neuropsychological evaluation; implying that she was mentally impaired or…
- AHRP Speaks Out | Conflict of Interest | Current Controversies | Medical Safety Issues | Pharma Corrupt Influence | Public Policy | Vaccine Risks
CDC Measles Vaccination Rule Has Left Millions of Vaccinated Americans Without Protection
Lots of babies get measles in Africa and India, and it is a significant cause of death there. A great deal of work has gone into developing measles vaccines that can be given to children at younger and younger ages, especially in Africa, for this reason. But in the United…
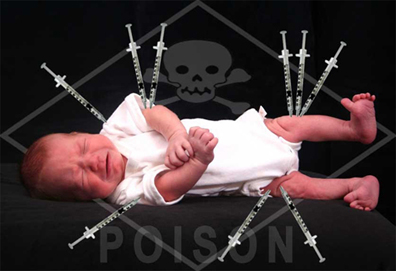
Childhood Vaccines that are Unjustifiably Mandated
Below, Meryl Nass, MD,* an internal medicine physician whose expertise in vaccines includes creating a methodology for investigating and distinguishing between natural epidemics and biological warfare events, provides substantive information about several vaccines that are on the Childhood Vaccination Schedule. Childhood Vaccines that are Unjustifiably Mandated Meryl Nass, MD February 25,…
Media interviews Dr. Meryl Nass re: anthrax vaccine and anthrax attacks
Dr. Meryl Nass, a member of the board of directors of The Alliance for Human Research Protection (AHRP), is an acknowledged expert on anthrax, biological terrorism, and is a US authority on adverse reactions due to the anthrax vaccine. Dr. Nass has been a leading opponent of U.S. military policies that continue to treat military servicemembers as a ready pool of experimental subjects, in the absence of meaningful informed consent.
Dr. Nass will be appeaing on several national and international TV and radio programs:
 AHRP Speaks Out | Board of Directors | Corporate-Corrupted Medicine | Corrupt Practices | Corrupt Public Health | Corrupted Science | Covid Pandemic | Current Controversies | Fear-Propaganda | Global Public Health Institutions | Government Overreach | Involuntary Treatment | Medical Ethics - Human Rights | Propaganda -- Censorship | Vera Sharav, AHRP Board Member
AHRP Speaks Out | Board of Directors | Corporate-Corrupted Medicine | Corrupt Practices | Corrupt Public Health | Corrupted Science | Covid Pandemic | Current Controversies | Fear-Propaganda | Global Public Health Institutions | Government Overreach | Involuntary Treatment | Medical Ethics - Human Rights | Propaganda -- Censorship | Vera Sharav, AHRP Board MemberVera Sharav Statement Against Efforts by the World Health Organization to Dictate Global Health Policy
Stop the World Health Organization Treaty that would dictate global public health policy — in other words, the Treaty would override national sovereignty. Deadline for submission — 18 hours. My submission: There are a wide range of problems with the Pandemic Treaty. First and foremost is the World Health Organization’s…
